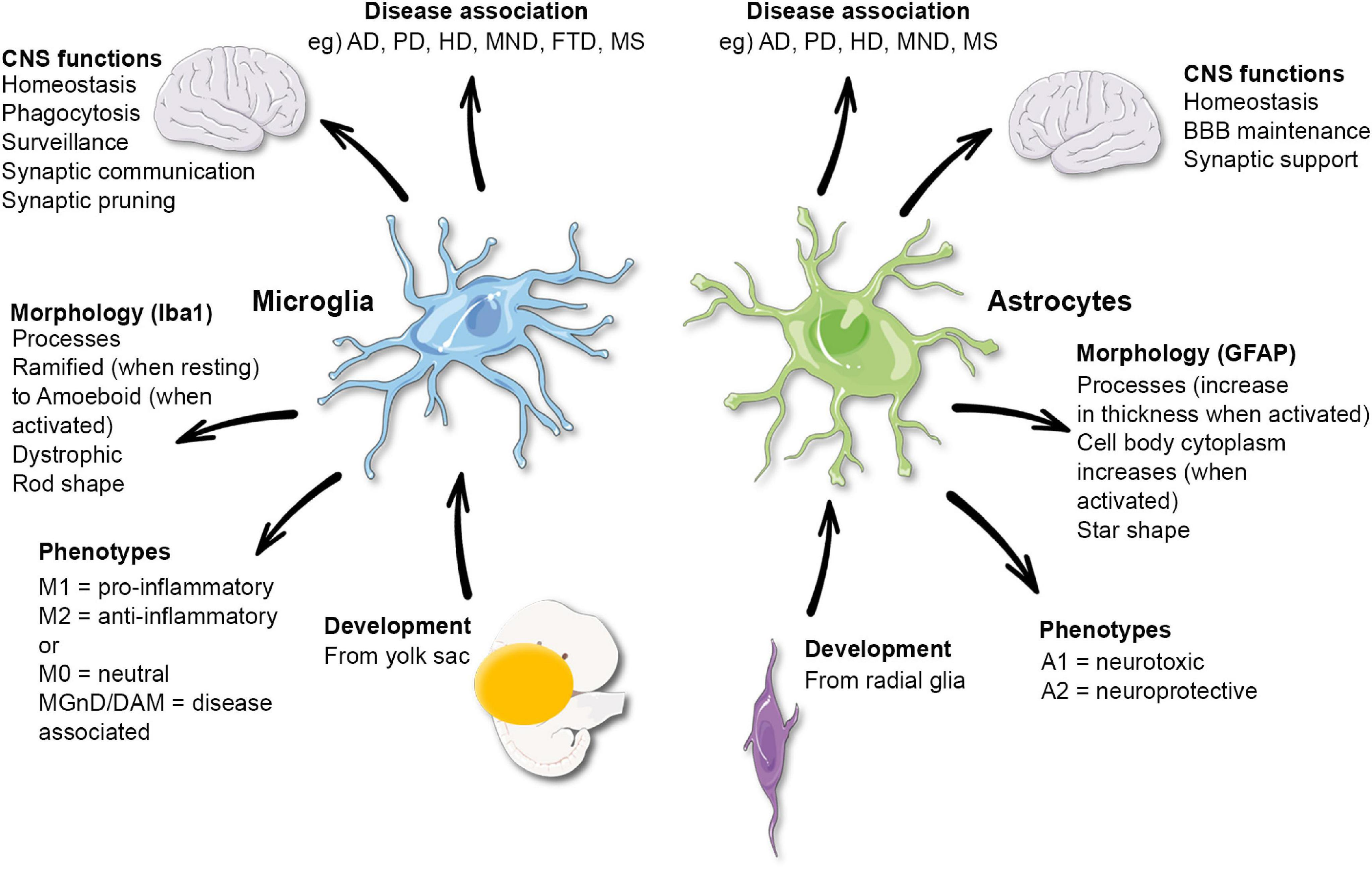
Microglial research is at the forefront of understanding the brain’s immune system and its critical role in neurodegenerative disorders such as Alzheimer’s disease. Pioneered by scientists like Beth Stevens, this emerging field explores how microglia monitor and maintain brain health by clearing out damaged cells and pruning synapses. As our knowledge expands, it becomes clear that dysregulated microglial activity can lead to harmful effects, contributing to diseases like Huntington’s and other neurodegenerative conditions. By unraveling these complex mechanisms, Stevens and her team aim to identify new biomarkers and develop therapies that could revolutionize treatment for the millions affected by Alzheimer’s. This dynamic area of research not only deepens our understanding of brain function but also illuminates potential pathways for innovative medical solutions.
Exploring the dynamics of glial cells, specifically their role in the brain’s protective mechanisms, unveils a crucial aspect of neuroscience research. Often referred to as the brain’s immune response, these cells are essential for monitoring neural health and managing synaptic connections. Recent investigations, particularly in the context of Alzheimer’s and similar conditions, have highlighted the significance of these immune cell interactions. Scholars like Beth Stevens have made substantial contributions to this body of work, focusing on how microglial behavior correlates with the progression of neurodegenerative diseases. The findings pave the way for breakthrough discoveries in neurobiology, ultimately striving to enhance patient care for those battling these debilitating disorders.
Understanding Microglial Functions in Alzheimer’s Disease
Microglial cells play a crucial role in maintaining brain health by acting as the primary immune defenders of the central nervous system. In the context of Alzheimer’s disease and other neurodegenerative disorders, the functions of microglia extend beyond mere surveillance; they actively participate in synaptic pruning, a process that is essential for cognitive function. However, when this process goes awry, microglia can contribute to the pathological landscape of diseases like Alzheimer’s. Aberrant synaptic pruning has been implicated in the loss of synapses and neuronal death, leading to cognitive decline. Therefore, understanding the dual nature of microglial functions — protective versus destructive — is paramount in developing potential therapeutic strategies for Alzheimer’s patients.
Recent research led by Beth Stevens emphasizes how microglial dysfunction can exacerbate the progression of neurodegenerative diseases. By characterizing the conditions under which microglia engage in harmful synaptic pruning, scientists are uncovering new therapeutic targets. This research underscores the significance of the brain’s immune system in combating diseases like Alzheimer’s, ultimately shedding light on how targeted interventions could restore normal microglial activity and potentially preserve cognitive function in affected individuals. Through this ongoing research, there is a hopeful prospect for new biomarkers and treatments that could alter the course of Alzheimer’s disease.
The Role of Synaptic Pruning in Neurodegenerative Disorders
Synaptic pruning, a crucial process in brain development and function, can become detrimental when associated with neurodegenerative diseases. During normal brain development, microglia assist in eliminating excess synapses, ensuring the proper functioning of neural circuits. However, in Alzheimer’s disease, abnormal synaptic pruning can lead to synapse loss, contributing significantly to cognitive decline. Beth Stevens and her team have highlighted the importance of understanding these mechanisms, as they provide insight into the pathological alterations that occur in the brains of patients suffering from Alzheimer’s and similar disorders.
Ongoing studies into synaptic pruning reveal a complex interplay between microglial activity and neurodegenerative processes. By elucidating how microglia misregulate synaptic pruning, researchers can begin to identify potential interventions that could restore balance. Targeting the molecular pathways involved in microglial activation and synaptic pruning may provide a novel approach to mitigating the effects of Alzheimer’s disease. As Stevens points out, this line of research not only opens doors for new treatment strategies but also deepens our understanding of the brain’s immune system and its implications for overall neurological health.
Beth Stevens’ Transformational Research on Microglia
Beth Stevens has played a pivotal role in transforming the scientific community’s understanding of microglial cells and their functions. Her innovative research highlights how microglia, often overlooked as mere custodians of the brain, are key players in neurodevelopment and disease. By focusing on their role in synaptic pruning, Stevens has positioned microglial research at the forefront of Alzheimer’s disease investigations, demonstrating that these cells can both protect and damage neuronal networks. The implications of her findings extend beyond Alzheimer’s to various neurodegenerative disorders, illustrating the importance of microglial health in maintaining overall brain function.
Through extensive funding support, largely from the National Institutes of Health, Stevens has been able to advance her investigations into the mysteries of the brain’s immune system. Her work emphasizes the critical need for basic science in understanding complex disorders like Alzheimer’s. By unlocking the secrets of microglial function, Stevens and her colleagues are laying the groundwork for groundbreaking advancements in diagnostic and therapeutic approaches. The push for deeper insights into microglial behavior represents a significant stride towards ameliorating the devastating impacts of neurodegenerative diseases.
Implications of Aberrant Microglial Activity in Alzheimer’s
Aberrant microglial activity has emerged as a key factor in the progression of Alzheimer’s disease. As the brain’s immune system, microglia are typically responsible for clearing away harmful substances and supporting neuronal health. However, in Alzheimer’s patients, these cells can lose their regulatory functionality, leading to excessive synaptic pruning and inflammation, ultimately contributing to the neurodegenerative process. Understanding this dichotomy of microglial behavior is vital for researchers working to develop new treatments that harness the protective aspects of microglia while mitigating their harmful effects.
The findings from Stevens’ research underscore the urgency of targeting microglial dysfunction in Alzheimer’s therapy. By focusing on the molecular mechanisms that drive microglial misbehavior, researchers aim to devise interventions that restore their normal functions. This line of inquiry not only enhances our comprehension of Alzheimer’s pathology but also sets the stage for innovative treatment modalities. The anticipation surrounding these advancements highlights the potential for significant breakthroughs in supporting brain health and prolonging cognitive longevity in Alzheimer’s sufferers.
The Future of Microglial Research and Its Impact
As the field of microglial research continues to evolve, the implications for treating neurodegenerative disorders, particularly Alzheimer’s, are promising. With scientists like Beth Stevens at the helm, the focus on understanding microglial dynamics is reshaping therapeutic strategies. By delving deeper into how microglia interact with neurons and influence synaptic health, researchers hope to identify critical intervention points that could slow the progression of Alzheimer’s disease and enhance patient care. The future direction of this research is likely to yield novel biomarkers that can aid in the early detection of Alzheimer’s, offering hope for more effective treatments.
Additionally, the integration of advanced technologies, such as single-cell RNA sequencing and imaging techniques, is facilitating a more granular understanding of microglial functions in the elderly population. As these technologies proliferate, they promise to unveil the complex interplay between microglia, neurons, and other brain cells in the context of Alzheimer’s disease. By fostering collaborations across various disciplines and securing ongoing funding, researchers aim to accelerate discoveries that could lead to groundbreaking treatments, revolutionizing how we approach neurodegenerative conditions like Alzheimer’s.
Connecting Basic Science to Clinical Applications
The journey from basic scientific research to clinical application is often long and winding, particularly in complex fields like neurobiology. Beth Stevens exemplifies this pathway through her research on microglia and their influence on neurodegenerative disorders such as Alzheimer’s disease. Her work underscores the importance of foundational research, where seemingly esoteric studies can ultimately inform clinical needs and lead to novel therapeutic strategies. The synergy between curiosity-driven science and clinical relevance is crucial for advancing our understanding and treatment of Alzheimer’s.
In Stevens’ view, the complexities of the brain’s immune system are not only academic inquiries but hold the promise of real-world impact. By building upon the basic understanding of microglial roles and their effects on synaptic integrity, researchers can explore avenues for drug development that address the underlying mechanisms of Alzheimer’s disease. The transition from bench to bedside is paved by the insights gained from basic science, confirming that thorough exploration in research settings is key to translating findings into effective treatments for patients suffering from neurodegenerative disorders.
The Importance of Federal Funding in Neuroscience Research
Federal funding plays a pivotal role in supporting innovative research in the neuroscience field. Beth Stevens, advocating for the importance of federal grants, highlights how critical such funding has been in advancing our understanding of microglial cells and their relevance to Alzheimer’s disease. Government funding not only provides the financial resources necessary for high-risk research but also endorses the scientific exploration that may not attract immediate commercial interest. By investing in basic science, agencies like the National Institutes of Health enable researchers to pursue novel ideas with the potential for significant societal impact.
Moreover, consistent federal backing fosters a stable research environment conducive to long-term studies that can yield transformative findings. For Stevens and many of her contemporaries, this support has been instrumental in allowing their laboratories to operate and thrive. The long-term benefits of such investments can lead to substantial advancements in understanding neurodegenerative diseases and developing potential treatments. As the landscape of Alzheimer’s research evolves, continued advocacy for robust federal funding is essential to maintain momentum in addressing the challenges posed by Alzheimer’s and other neurodegenerative disorders.
Advancements in Early Detection of Alzheimer’s Disease
The early detection of Alzheimer’s disease is crucial for effective intervention and treatment. Advances in microglial research, particularly the work led by Beth Stevens, highlight potential biomarkers that could signal the onset of neurodegenerative changes long before clinical symptoms manifest. By understanding the role of microglia in synaptic health and dysfunction, researchers are identifying specific molecular signatures that may indicate early pathological processes associated with Alzheimer’s disease. Such breakthroughs are instrumental in shifting the paradigm from reactive to proactive approaches in treating neurodegenerative disorders.
Utilizing these insights, researchers aim to develop diagnostic tools that can facilitate earlier diagnosis and more personalized treatment plans for patients at risk of developing Alzheimer’s disease. These advancements reflect a growing recognition of the need for early-stage intervention strategies that could delay or mitigate cognitive decline. The commitment to elucidating the relationship between microglial behavior and Alzheimer’s pathology underscores a comprehensive approach that combines basic research findings with clinical objectives to enhance patient care.
The Future of Neurodegenerative Disease Research
Looking ahead, the future of neurodegenerative disease research appears bright as scientists build upon foundational studies like those conducted by Beth Stevens. The integration of interdisciplinary approaches, including genetics, molecular biology, and advanced imaging technologies, is paving the way for new discoveries that could revolutionize our understanding and treatment of Alzheimer’s disease and other neurodegenerative disorders. These advancements not only enhance our comprehension of pathogenic mechanisms but also foster the development of targeted therapies that address the root causes of these diseases.
The journey towards innovative treatments also requires a community of researchers dedicated to collaboration and knowledge-sharing. As insights from microglial research continue to unfold, the scientific community is encouraged to work together to translate these findings into actionable strategies for patient care. By emphasizing the importance of understanding the brain’s immune system and its intricate roles in neurodegenerative conditions, we can foster a research environment ripe for breakthroughs that will significantly improve outcomes for those affected by Alzheimer’s disease and other related disorders.
Frequently Asked Questions
What is the role of microglial research in understanding Alzheimer’s disease?
Microglial research plays a critical role in understanding Alzheimer’s disease as these brain immune cells are responsible for synaptic pruning and clearing out damaged neurons. Aberrant microglial activity has been linked to the progression of Alzheimer’s, making this research vital for developing new biomarkers and treatments.
How do microglia influence neurodegenerative disorders beyond Alzheimer’s?
Microglia influence various neurodegenerative disorders, including Huntington’s disease, by regulating the brain’s immune response. Their role in synaptic pruning can become dysfunctional, leading to increased neuroinflammation and neuronal damage, furthering the pathology of diseases like Alzheimer’s and Huntington’s.
What are the implications of microglial research for the brain’s immune system?
Microglial research highlights the importance of the brain’s immune system in maintaining neural health. By understanding how microglia regulate synaptic connections and respond to brain injury, researchers can uncover new therapeutic targets to combat neurodegenerative disorders such as Alzheimer’s.
How does Beth Stevens’ work advance microglial research?
Beth Stevens’ work significantly advances microglial research by demonstrating the dual role of microglia in normal development and disease. Her research at the Stevens Lab focuses on the mechanisms behind synaptic pruning by microglia and how their dysfunction may contribute to Alzheimer’s and other neurodegenerative disorders.
What findings have emerged from studying microglia in relation to synaptic pruning?
Studies of microglia in relation to synaptic pruning have revealed that these cells are essential in shaping brain connectivity. Aberrant synaptic pruning conducted by microglia can lead to neurodegeneration, particularly in Alzheimer’s disease, emphasizing the need for targeted research in this area.
What challenges does microglial research face in neurodegenerative disease treatment?
Microglial research faces challenges such as the complexity of brain immune responses and the difficulty in translating basic science findings into clinical applications for neurodegenerative diseases like Alzheimer’s. Understanding the nuanced role of microglia in synaptic pruning continues to be a key focus for overcoming these challenges.
How can microglial activity be measured or targeted for Alzheimer’s disease therapies?
Microglial activity can be measured using advanced imaging techniques and biomarkers. Targeting microglia for Alzheimer’s disease therapies involves modifying their function to enhance protective roles or inhibit harmful activity, as shown in promising studies emanating from research led by scientists like Beth Stevens.
What future research directions are anticipated in microglial studies related to neurodegenerative diseases?
Future research in microglial studies related to neurodegenerative diseases like Alzheimer’s may focus on identifying specific pathways involved in microglial dysfunction, developing targeted therapeutics, and understanding how these immune cells interact with neurons during the disease progression.
| Key Point | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune system, clearing dead cells and pruning synapses. |
| Link to Alzheimer’s Disease | Aberrant synaptic pruning by microglia is implicated in Alzheimer’s and other neurodegenerative disorders. |
| Impact on Research | Innovative research led to potential biomarkers and treatment strategies for neurodegenerative diseases, benefitting millions. |
| Funding Support | Research has been significantly supported by NIH and other federal funding, bolstering progress in microglial research. |
| Curiosity in Science | Stevens emphasizes the importance of basic, curiosity-driven science in advancing our understanding of microglia. |
Summary
Microglial research is essential for understanding the brain’s immune system and its role in neurodegenerative diseases such as Alzheimer’s. The advancements made by researchers like Beth Stevens highlight the importance of foundational science in uncovering the complexities of brain health. By addressing how microglia interact with neurons, this research not only enhances our understanding of disease mechanisms but also paves the way for innovative treatments. As the field progresses, it holds promise for significantly improving care for millions affected by Alzheimer’s and similar diseases.